CLINICAL TRIAL RESULTS DEMONSTRATE NUEDEXTA EFFICACY

NUEDEXTA Can Help Reduce Pseudobulbar Affect (PBA) Episodes After 90 Days.1-3,7
THE STAR PIVOTAL TRIAL – For Patients with ALS and MS
NUEDEXTA significantly reduced PBA episode rates compared with placebo in patients with amyotrophic lateral sclerosis (ALS) or multiple sclerosis (MS).1,2

The STAR Pivotal clinical trial was a randomized, placebo-controlled study of 326 patients researching NUEDEXTA’s efficacy.1,2
A change in the number of PBA episodes per day was the primary endpoint of the Pivotal clinical trial. Patients on NUEDEXTA experienced a 3.9 mean reduction in the daily PBA episode rate (6.8 baseline) versus a 3.0 mean reduction (4.5 baseline) for those on placebo (P=0.005 versus placebo).1,2
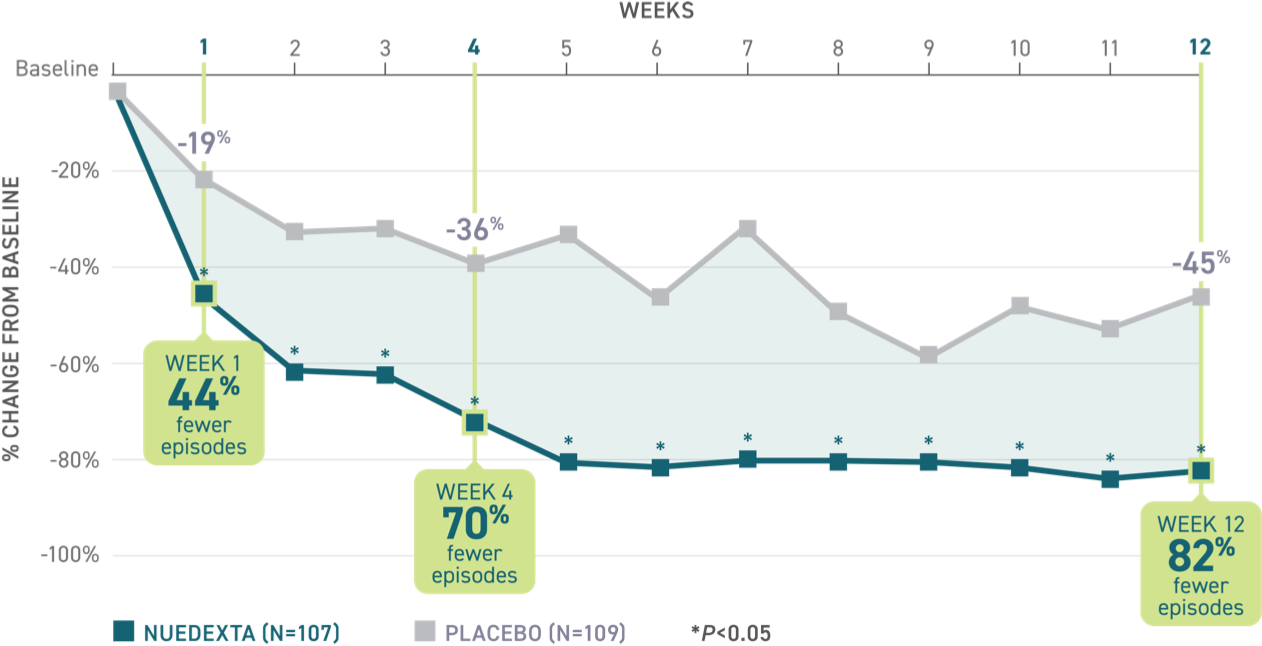
Post hoc analysis: Episode rate reduction from baseline in the 12-week Pivotal clinical trial3
Over 90 days, patients on NUEDEXTA experienced fewer PBA episodes, with a reduction of 82% at Week 12. Patients received NUEDEXTA once daily in Week 1 and once every 12 hours starting at Day 8.2
Secondary outcomes showed significant improvement with NUEDEXTA versus the placebo2
At Week 12, the mean Center for Neurologic Study-Lability Scale (CNS-LS) score decreased by 8.2 points for patients on NUEDEXTA from a 21.0 baseline versus 5.7 points for patients on placebo from a 19.9 baseline (P=0.0113).1,2
*The CNS-LS is a seven-item self-report rating scale that measures perceived frequency and control over laughing and/or crying episodes. It was validated as a PBA screening tool in ALS and MS. A CNS-LS score of 13 or greater may suggest PBA but does not confer a PBA diagnosis.5,6
51% OF PATIENTS ACHIEVED REMISSION
Complete episode remission was achieved by 51% of patients on NUEDEXTA compared with 29% of patients on a placebo (P<0.005).2
Remission was defined as the absence of PBA episodes during the patient’s final 14 days of participation in the 12-week study.2
THE PRISM II OPEN-LABEL STUDY – For Patients with Stroke, Dementia, and TBI

PRISM II was an open-label trial of 367 patients. Change in CNS-LS score was the primary endpoint.7
NUEDEXTA was effective in reducing PBA symptom scores and episode rates in patients with stroke, dementia, and TBI.7
The CNS-LS score in all cohorts combined decreased from 20.4 at baseline to 12.8 at Day 90 (P<0.001 versus baseline). CNS-LS reductions were similar across the dementia, stroke, and TBI cohorts, with a range of 7.2 to 8.5 at Day 90.7
Secondary endpoint: median change in number of PBA episodes per week from baseline7-10
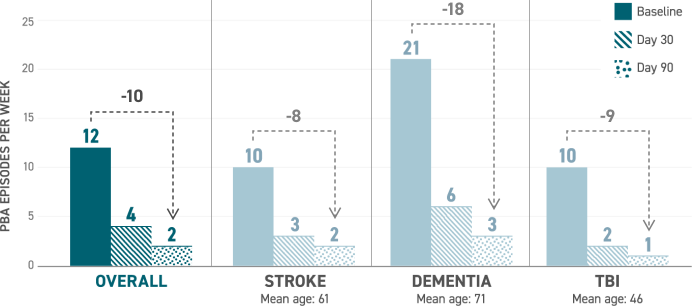
Patients in the study experienced a sizable decrease in the number of PBA episodes per week after 90 days. Overall, patients started with about 12 episodes per week at baseline, 4 episodes by day 30, and 2 by day 90.
Interested in how to prescribe NUEDEXTA? Check out our Dosing and Treatment page.





