NUEDEXTA’S SAFETY PROFILE

NUEDEXTA Side Effects
NUEDEXTA has a demonstrated safety profile. Adverse events with NUEDEXTA were generally mild to moderate in nature and consistent across studies.1
Common adverse events in the STAR Pivotal Trial1,2,a
aAdverse effects occurring in 5% or more of patients on NUEDEXTA and at two times or more the placebo rate.
Adverse effects leading to discontinuation1,2
The most commonly reported adverse reactions (incidence ≥2% and greater than placebo) that led to discontinuation of NUEDEXTA were muscle spasticity (3%), respiratory failure (1%), abdominal pain (2%), asthenia (2%), dizziness (2%), fall (1%), and muscle spasm (2%).
Risk of falls1
- The incidence of falls with NUEDEXTA was similar to placebo; however, NUEDEXTA may cause dizziness.
- Precautions to reduce the risk of falls should be taken, particularly for patients with motor impairment affecting gait or a history of falls.
These are not all the risks from use of NUEDEXTA. Learn More:
Adverse events in the PRISM II open-label study3,b
Reported adverse events were generally consistent with the NUEDEXTA safety profile observed in the placebo-controlled Pivotal trial.1-3
bAdverse events occurring in more than 1% of patients.
NUEDEXTA Drug Interactions
NUEDEXTA may be taken with other medications, but there is a risk for adverse drug interactions. Remind your patients to talk to all their healthcare providers about any medications they are taking, including NUEDEXTA, and any side effects that they experience.
Is your patient also being treated for a mood disorder such as depression?
PBA is often comorbid with depression and other mood disorders, but PBA is a distinct condition that should be treated separately.3,6 Patients with PBA may already be on treatment for other conditions. In the PRISM II study, nearly 71% of participants (n=260) were on psychopharmacologic medications, including anticonvulsants, antipsychotics, antidepressants, sedatives/hypnotics or anxiolytics, and benzodiazepines.3
NUEDEXTA is not an antipsychotic medication and is classified as “Central Nervous System, Other” in the United States Pharmacopeia and National Formulary (USP-NF).9
When prescribing NUEDEXTA for patients with PBA, keep in mind that it should not be taken with monoamine oxidase inhibitors (MAOIs) or by patients who have taken MAOIs in the last 14 days.1 While NUEDEXTA may be taken with some selective serotonin reuptake inhibitors (SSRIs) or tricyclic antidepressants, it increases the risk for serotonin syndrome.1 For dosing precautions for SSRI paroxetine and tricyclic antidepressant desipramine, review Section 12.4 of the full prescribing information linked below.
These are not all the possible risks, drug interactions, or side effects associated with NUEDEXTA. Learn more:
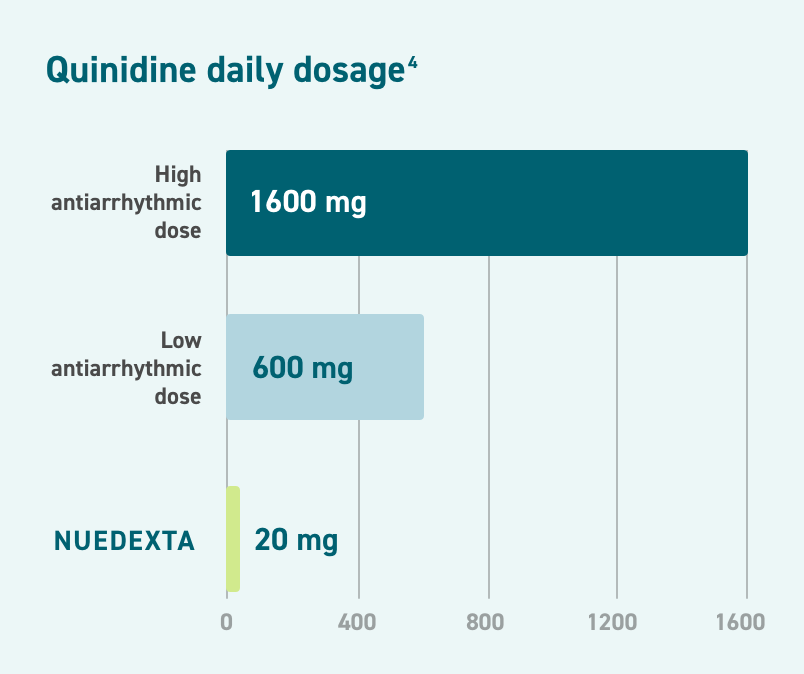
NUEDEXTA Contains an Ultra-Low Dose of Quinidine.1
The daily quinidine dose (20 mg daily, 10 mg every 12 hours) in NUEDEXTA is about 3% of the lowest recommended antiarrhythmic dose.4
CARDIOVASCULAR CONTRAINDICATION1
NUEDEXTA is contraindicated in patients with a prolonged QT interval, congenital long QT syndrome, history suggestive of torsades de pointes, heart failure, patients receiving drugs that both prolong QT interval and are metabolized by CYP2D6 (e.g., thioridazine and pimozide), patients with complete atrioventricular (AV) block without implanted pacemaker or at high risk of complete AV block.
Learn about NUEDEXTA’s dosing guidelines on our Dosing and Treatment page.





