NUEDEXTA SAFETY & EFFICACY

NUEDEXTA is the first and only FDA-approved treatment for Pseudobulbar Affect (PBA)1
NUEDEXTA is clinically proven to reduce PBA episodes and has a demonstrated safety profile.1,3,5
PBA causes uncontrollable laughing and/or crying that happens suddenly. Because episodes are unpredictable, many patients worry about having an episode in public — and they may feel uncomfortable in social settings. But the disorder is manageable and NUEDEXTA can help reduce their daily episodes.1,2
Reducing PBA episodes can make a difference.
For patients with PBA, each episode can have an impact. In one web-based survey (N=1,052) of patients with an underlying neurologic condition associated with PBA or their nonpaid caregivers, respondents who described their uncontrollable laughing and/or crying episodes as “extremely” or “very” burdensome had experienced an average of 8.8 crying episodes or 4.6 laughing episodes in the past week.8 Patients with PBA symptoms (N=399) were defined as those who scored 13 or greater on the Center for Neurologic Study-Lability Scale (CNS-LS).*
*The CNS-LS was validated as a measure of perceived episode frequency in amyotrophic lateral sclerosis and multiple sclerosis populations. The scale does not confer a PBA diagnosis.6,7
NUEDEXTA’s Safety And Efficacy Were Evaluated In Two Clinical Trials
The STAR Pivotal trial was a phase 3, double-blind, placebo-controlled study (N=326) in patients with PBA secondary to amyotrophic lateral sclerosis (ALS) or multiple sclerosis (MS).3
NUEDEXTA significantly reduced PBA episode rates compared with placebo in the 12-week Pivotal trial5
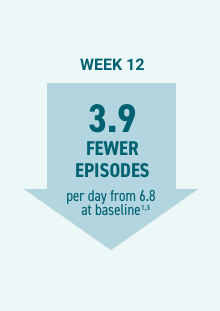
Efficacy:
- At Week 12, patients on NUEDEXTA experienced a 3.9 mean reduction in the daily PBA episode rate (6.8 baseline) versus a 3.0 mean reduction (4.5 baseline) for those on placebo (P=0.005).1,3
- In a post-hoc analysis, NUEDEXTA significantly reduced PBA episodes rates compared to placebo starting at Week 1.
- Complete PBA episode remission was achieved by 51% of patients on NUEDEXTA versus 29% on placebo by Week 12 (P<0.005).3 Remission was a secondary outcome, defined as the absence of PBA episodes during the patient’s final 14 days of participation in the 12-week study.
Safety:
- The most common adverse effects were diarrhea and dizziness.1,3

The PRISM II study was a phase 4, open-label study (N=367) in patients with PBA secondary to stroke, dementia, or traumatic brain injury (TBI).4
NUEDEXTA reduced PBA symptom scores and episode rates in patients with stroke, dementia, and TBI.5
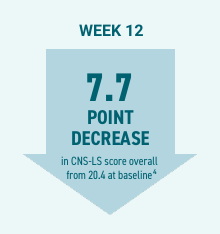
Efficacy:
- At Day 90 of the PRISM II study, patients’ mean Center for Neurologic Study-Lability Scale (CNS-LS) score decreased by 7.7 points from 20.4 at baseline (P<0.001).4
- Overall, patients started with about 12 episodes per week at baseline and 2 by Week 12.4
Safety:
- The most common adverse effects were diarrhea and headache.1,4
Safety and Efficacy Pocket Guide
Keep NUEDEXTA’s safety and efficacy profile handy with a pocket guide. Download Now
NUEDEXTA is the first and only FDA-approved treatment for PBA.1 Explore the pages below to learn more about treating PBA with NUEDEXTA.

Efficacy
Review the efficacy data from NUEDEXTA’s clinical studies.

Safety
Read about NUEDEXTA’s safety profile.

Dosing
Learn how to prescribe NUEDEXTA for your patients with PBA.





